Measuring Principle
What is Non-Dispersive Infrared Absorption Method (NDIR)?
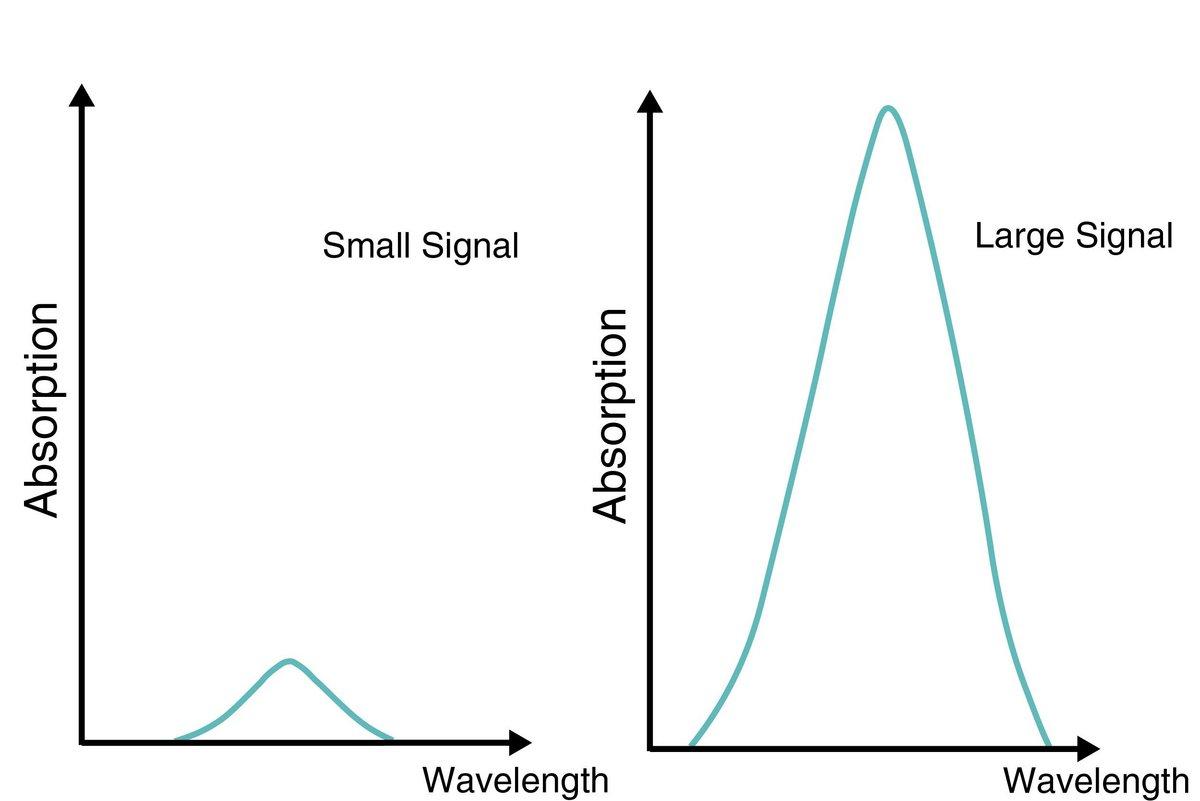
Non-dispersive infrared absorption (NDIR) is a method using infrared absorption of molecules.This method is used to measure the concentration of various gas components that actively absorb infrared.
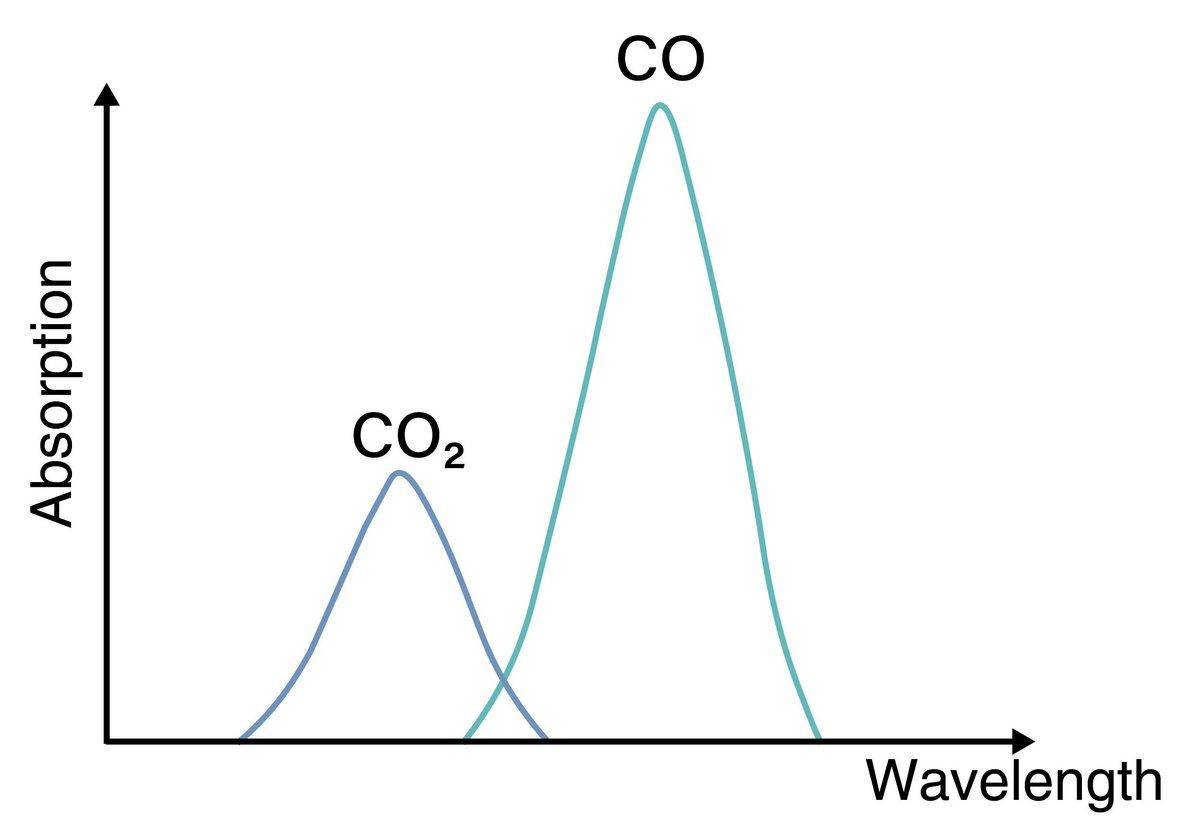
Molecules have inherent capability to selectively absorb light at specific wavelengths. For example, a red apple appears red because its surface absorbs light with wavelengths other than red and, conversely, reflects light with red wavelengths. This happens in infrared radiation, which is widely used to measure the concentration of gas components. When infrared radiation is absorbed by a molecule, it increases the temperature of that molecule. The wavelength of infrared radiation absorbed differs depending on the molecule (i.e. like a fingerprint, its wavelength profile is specific for each molecule).
Non-dispersive infrared absorption method (NDIR) utilizes this property, the term NDIR has also been long established. Infrared radiation is classified into near-infrared rays, mid-infrared rays, and far-infrared rays corresponding to wavelength. NDIR uses infrared radiation containing wavelengths in mid-infrared radiation of 2.5-25μm to measure the concentration of gas components.
A non-dispersive type is a measurement method which uses specific infrared radiation wavelength in mid-infrared emitted from an infrared light source. Some gases do not absorb infrared radiation. For example, nitrogen (N2) does not absorb infrared radiation, so it is measured by a gas analyzer using a different measuring principle.